PT. Kalmed Sejahtera Indonesia (KSI) has the experience in handling licenses of Medical device distribution for domestic and overseas clients.
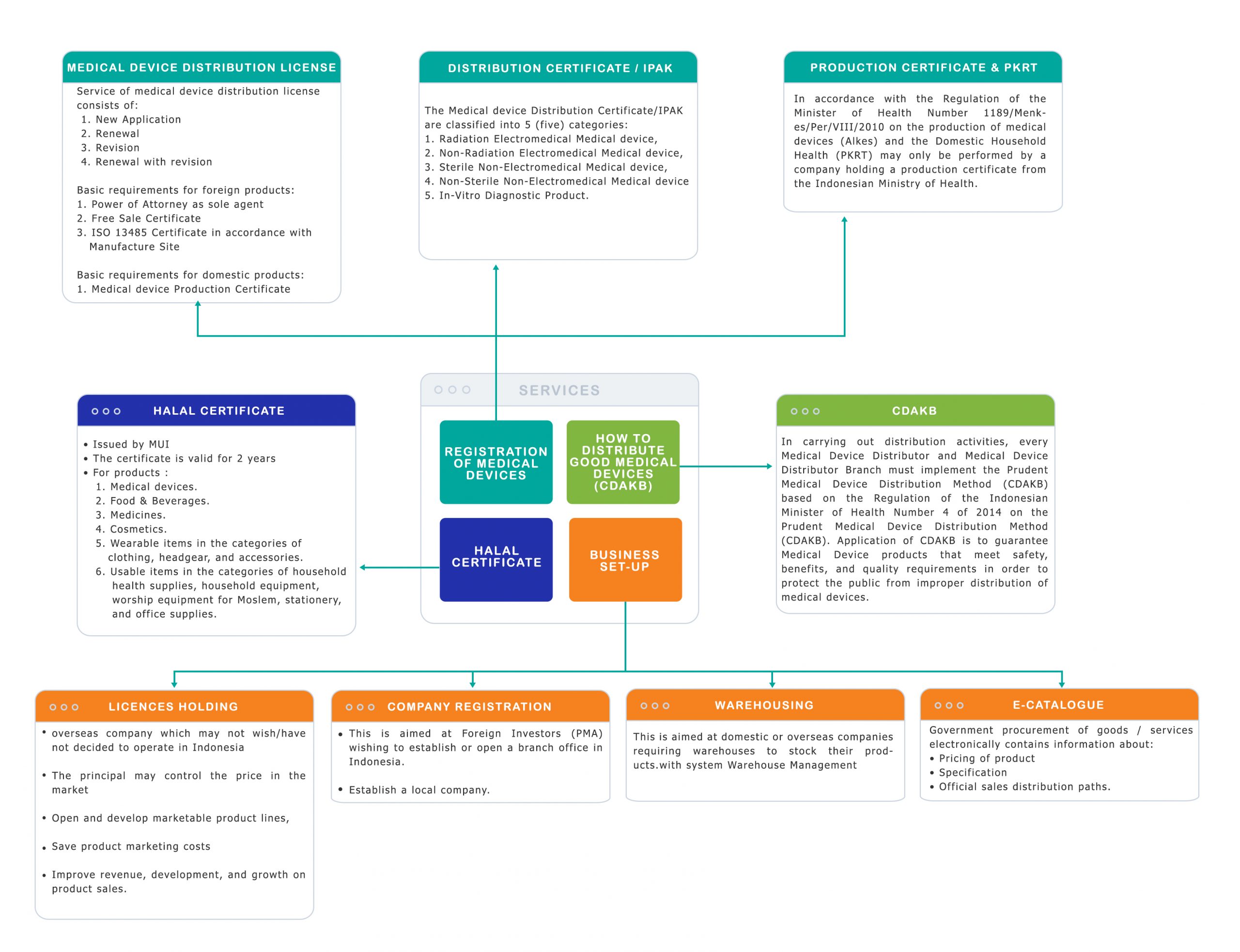
The scope of our service is Regulatory Consultancy covering distribution license for Health Devices to the Ministry of Health, license holder of medical device distribution including management of imported products, E-catalog, Building Rental, Halal Certificate, Registration Certificate (STP), and Prudent Medical Device Distribution Method (CDAKB).
KSI employs professional staffs to serve and ease your business sector to function competently in the fulfilling of service we offer in the field of medical devices and other related fields.